This study is performed by Susanne Pors at the Laboratory of Reproductive Biology, Rigshospitalet, Denmark, in collaboration with Clinical Chemistry, Lund University, Sweden. Based on new knowledge from this project it is the goal, in the future, to implement a new method for assisted fertility treatment.
Background
Some women with reduced fertility cannot benefit from traditional assisted fertilization methods. Women with failing ovary functions due to age, sudden diseases with limited oocyte reserves or adults or pre-pubertal girls who must receive chemotherapy, need more specific methods to preserve and restore ovarian function with follicle activation and maturation.
Cryopreservation of ovarian tissue is an opportunity for these patients to maintain an option for pregnancy. Freezing the ovaries preserves the ability to restore ovarian function after transplantation. This method, however, has not yet reached its full potential. In order to increase the chances for later fertilization, additional handling of the ovarian tissue is needed.
The follicle activation process is not fully understood. The ovary contains follicles in many different development stages. The majority will be small and resting primordial follicles (see bottom picture), which contain an oocyte and surrounding granulosa cells. By a highly regulated irreversible process, a number of the primordial follicles are selected for growth and activation. Genomic and proteomic analysis have identified key paracrine and autocrine signaling pathways, which are associated with the first signs of primordial follicle activation. A number of the genes have been shown to exert both positive and negative effects on follicle activation. The development of a primordial follicle to a fully mature follicle with a competent oocyte takes about 5-6 months and is a complicated process, where a variety of hormones and growth factors play crucial roles.
Aim of the study
The aim of the project has been to study the earliest stages of ovarian follicle development to understand the mechanisms leading to follicular growth and to establish a safe and reproducible protocol for activation of human primordial follicles. I have made extensive work to establish the best possible protocol by using different model systems including mice and human ovarian tissue, culture in vitro and in vivo transplantation and isolated cells/follicles and tissue.
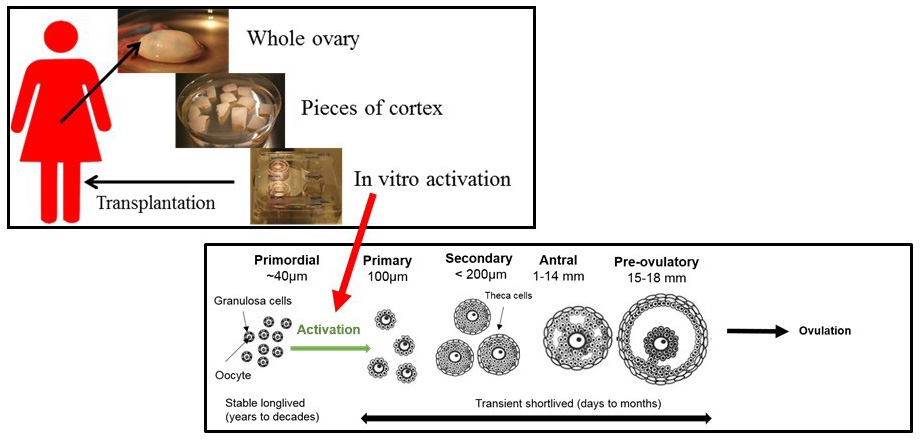
Preliminary results
The working methodology can be seen in the top picture where the cortex from the ovary is cut into small pieces. Thereafter the ovarian tissue has been treated with different chemicals in attempts to activate the follicles within the tissue and in total around 50 different treatments of tissue have been tested. Promising results have been made towards approaches using the compound S1P and use of a combination of the so-called mTOR activators.
In the future, when the method has been fully developed, the aim is to transplant the tissue back into the women which will restore the ovarian function and hopefully lead to regained and even increased fertility due to the increase in activated follicles.
