This study is performed by Lihua Dong at the Laboratory of Reproductive Biology, Rigshospitalet, Denmark in collaboration with Reproductive Medicine, Lund University, Sweden and Clinical Institute, Odense University Hospital, Denmark.
Patients undergoing chemotherapy for malignant disease will become sterile as a direct consequence of the treatment. For adult men fertility can be restored if sperm is preserved before treatment and later used for IVF. Pre-pubertal boys do not have the same opportunity. Today, no efficient method exists to preserve fertility for these patients. Approximately 100 and 150 boys in Denmark and Sweden require chemotherapy for cancers every year and 80 % of the boys survive to reach adult life where many of them wish to have a family.
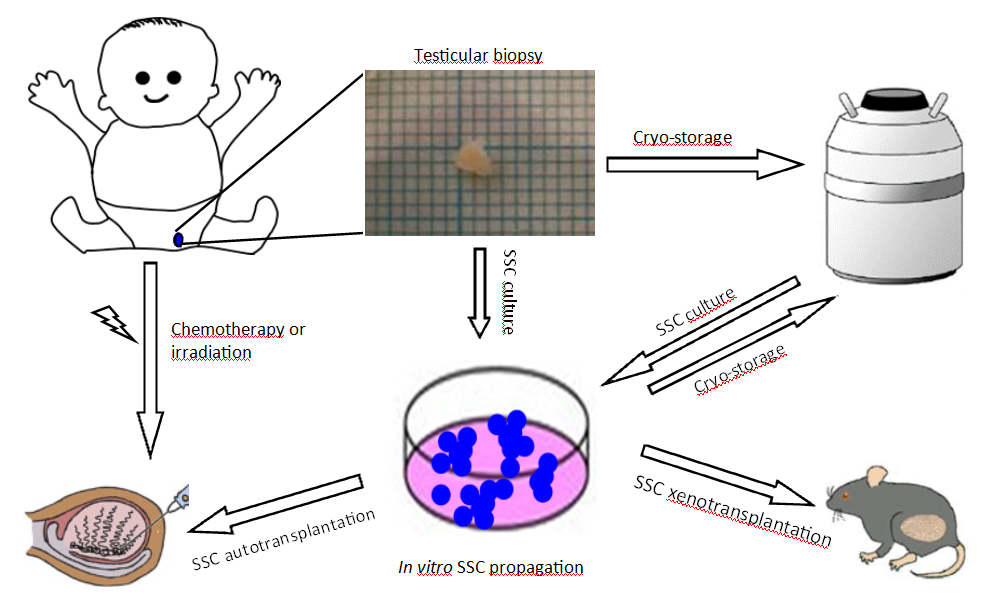
This project attempts to develop a new method for fertility preservation and restoration in pre-pubertal boys, as shown in the scheme. In a pre-pubertal patient with cancer or fertility-threatening disease, a testicular biopsy containing spermatogonial stem cells (SSCs) is taken before gonadotoxic treatment. The biopsy containing SSCs or SSCs after in vitro propagation could be cryopreserved. After successful cancer treatment, the SSCs would be autologously transplanted back to the patient’s testis to re-initiate spermatogenesis and restore fertility. Before SSC auto-transplantation, the human SSCs will be transplanted into immunodeficient mice to validate the SSC functionality and demonstrate no cancer cell contamination.
The project has cryopreserved testicular tissue from 17 patients. Due to the very small biopsies the cells need to be propagated in order to have sufficient amount of SSCs to transplant back to the patient to restore spermatogenesis. Lihua has managed with the very difficult task of consistently propagate SSCs, verified by staining for specific markers.
Thereafter, SSCs are transplanted into immunodeficient mice to demonstrate the functionality of the cells as well as secure that there are no cancer cells left which otherwise presents a current risk with transplantation therapy for the patients.
For patients with a dysfunction within the spermatogenesis the above-mentioned method is not enough to restore fertility. The last part of the study addresses ways of maturing SSC’s into spermatocytes. Lihua performs in vitro spermatogenesis experiments in a 3-D culture system with a modified media from in vitro spermatogenesis of mouse neonatal testis. The meosis process of SSCs is under investigation.
